20 Jan Galvanic Corrosion: A Preventable Problem
If you work with architectural metals, you have probably heard the term “galvanic corrosion.” But what is it, why is it a problem, and how can you prevent it? Let’s see if we can shed some light on the subject.
What Is Galvanic Corrosion?
Galvanic corrosion occurs when base metals (metals that tend to corrode) come into contact with noble metals (metals that tend to resist corrosion). With the help of an electrolytic component (i.e., contact with water), the chemical reaction between the two dissimilar metals creates an electrical current, which causes corrosion.
Which Metals Are Base, and Which Metals Are Noble?
When it comes to architectural metals, this list puts the most common metals in order from least corrosive (noble) to most corrosive (base):
- Stainless Steel (least corrosive – Noble)
- Copper, Brass, and Bronze
- Steel
- Aluminum
- Zinc (most corrosive – Base)
The Four Conditions For Galvanic Corrosion
In order for a noble metal to corrode a base metal, these four conditions must take place:
- Two dissimilar metals
- Direct or close contact between the metals
- A large difference in the galvanic potential between the two metals.
- A conductive electrolyte (usually water, but it could be soil or humid air) connects the metals.
Please keep these rules of thumb in mind when designing your metal parts. And if we can ever answer any questions during your design phase, please feel free to reach out to the team at Astro, and we will be glad to advise.
Not All Dissimilar Metals Cause A Galvanic Corrosion Issue
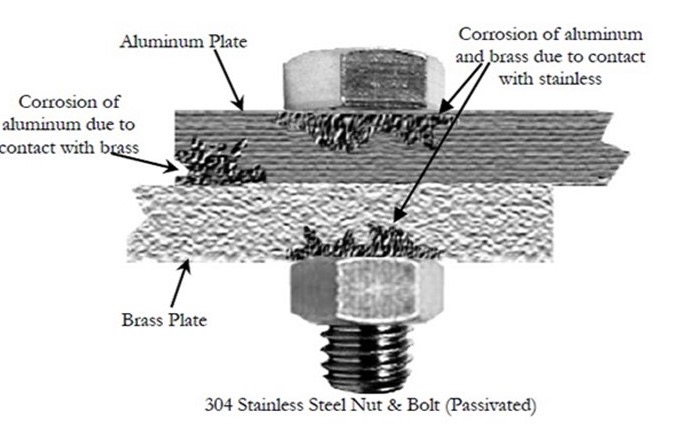
Metals close together on the table above exhibit almost no corrosion when placed together. For example, aluminum and zinc are very close in terms of their baseness, so there is relatively little risk of corrosion between these two metals.
Also, the larger the surface area of the contact between dissimilar metals, the less of a problem galvanic corrosion will be. For example, if a large sheet of stainless steel is sitting on top of a large sheet of aluminum, the rate of galvanic corrosion slows considerably, because the large surface area spreads out the current.
Galvanic corrosion most often presents a problem when to highly dissimilar metals touch on a relatively small surface area. For instance, if you attach stainless steel panels with zinc-coated screws, the stainless steel will attack the zinc-coated fasteners from all directions and will aggressively corrode the zinc. This can cause rust lines to run down the panels, and eventually, it can cause structural failure.
However, in our earlier example, zinc fasteners could be used to attach aluminum panels with minimal risk of corrosion because the two metals have similar potentials.
How Can Galvanic Corrosion Be Prevented?
- Use similar metals (metals close together on the nobility scale).
- Avoid contact with water.
- Avoid small base metals against large noble metals
- Use insulators or coatings to create physical separation between dissimilar metals
Please keep galvanic corrosion in mind when designing your architectural metals, to avoid big corrosion headaches down the road!